Case Study – Ramjet CFD Model
The challenge
A South African company is performing a series of tests on laboratory-based
ramjets. The tests involve different fuels, such as solid magnesium-based fuels
and liquid hydrocarbon fuels. The aim is to find a combination of fuel and
combustion chamber to achieve complete combustion of the fuel, uniform flow at the
outlet nozzle of the ramjet, and a high gross thrust. One test with gaseous JP10
fuel (C10H16) yields a measured gross thrust of 3106 N. The
company wishes to know whether this thust could have been predicted by a
computational fluid dynamics (CFD) model.
The solution
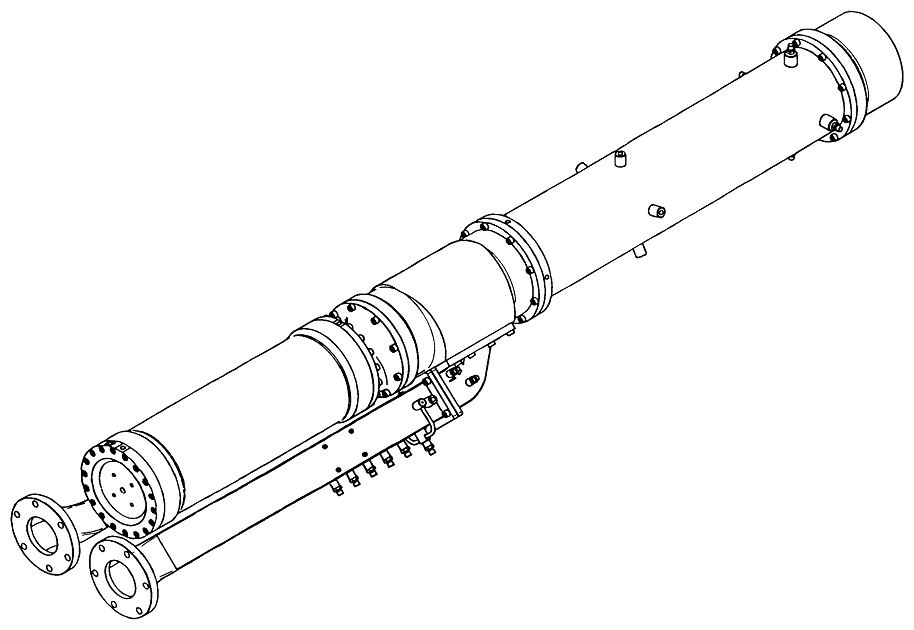
The experimental ramjet is shown in Figure 1. The combustion chamber of the ramjet
is fed with air through two ducts, each containing a converging-diverging nozzle.
The air flow chokes in the throat of the nozzles, and the area of the throat,
together with the total temperature and total pressure of the air, fixes the mass
flow of air entering the combustion chamber. The fuel injector at the head of the
combustion chamber is fed with a partially burnt mixture of 75% gaseous JP10 and
25% oxygen by mass. The fuel injector also contains a converging-diverging nozzle
which fixes the mass flow of hot combustion gases entering the combustion chamber.
When the air and the combustion gases mix in the combustion chamber the combustion
of the JP10 is completed. The combustion products leave the chamber through a
converging-diverging nozzle which takes the products up to supersonic speed.
Figure 1 Experimental ramjet
Atkinson Science created a CFD model of the ramjet. The ramjet is symmetrical about
the vertical plane between the two air ducts, so it was only necessary to model
one half of the ramjet. Consequently, the model includes the whole of one air duct
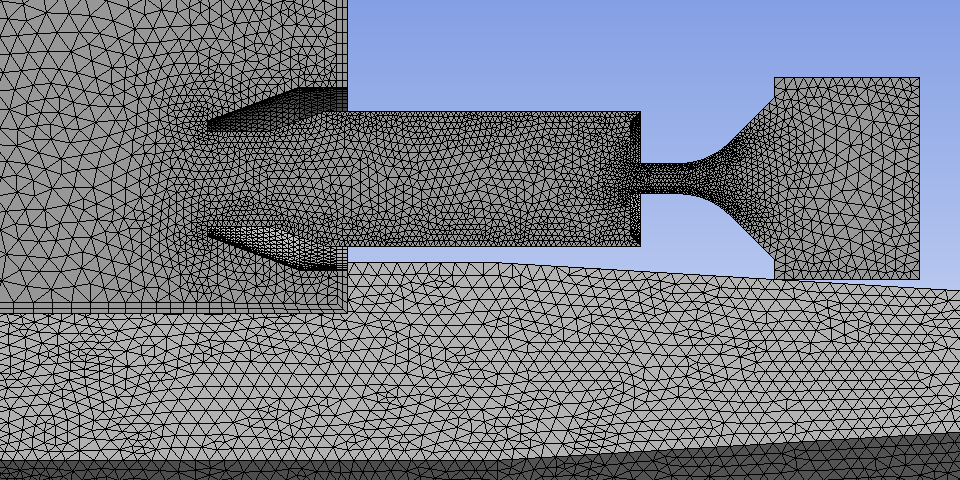
and one half of the fuel injector, combustion chamber and outlet nozzle. Figure 2
shows a detail of the surface mesh around the fuel injector.
Figure 2 Surface mesh around the fuel
injector
The combustion of the JP10-oxygen product mixture and the air can be modelled using
the eddy dissipation combustion model of Ref. [1]. In this model the rate of
burning of the reactants is determined either by the chemical kinetic rate or the
turbulent mixing rate, whichever is the slower. We used the three-step chemical
reaction mechanism and the kinetic rate constants proposed in Ref. [2]. This
reaction mechanism includes a dissociation step in which carbon dioxide reverts
back to carbon monoxide and oxygen and without which the combustion temperature
would be overpredicted. The turbulence was modelled with the widely-used
standard k-ε model.
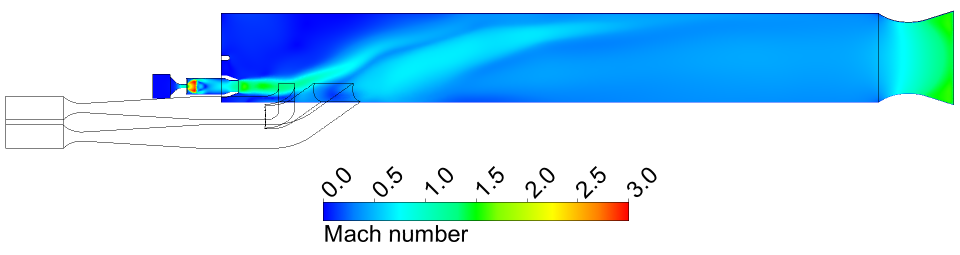
We found that the combustion chamber produced very efficient combustion. As shown
in Figure 3, all of the JP10 is consumed. Also, the temperature and Mach number at
the nozzle exit are very uniform, as shown in Figures 4 and 5, with average values
of 2060 K and 1.25, respectively. The calculated gross thrust (momentum flux from
the nozzle) is 3246 N, which is very close to the measured value of 3106 N.
Figure 3 JP10 mass fraction in the symmetry
plane of the ramjet
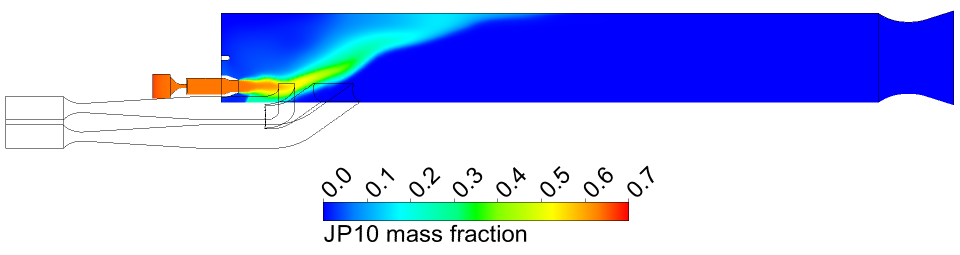
Figure 4 Temperature in the symmetry plane
of the ramjet
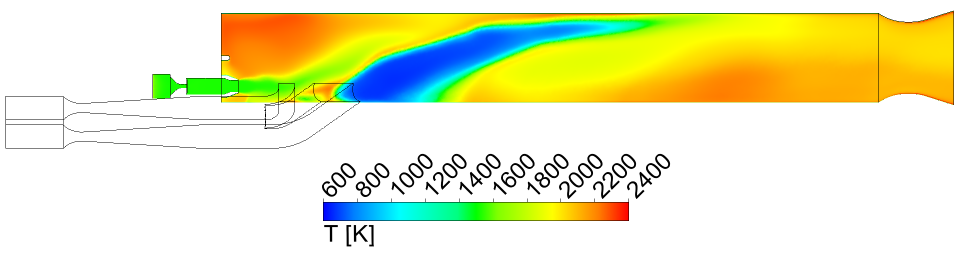
Figure 5 Mach number in the symmetry plane
of the ramjet
The benefits
The close agreement between computed and measured gross thrust shows that CFD
allied to comparatively simple turbulence and combustion models can be used with
confidence to predict the performance of a ramjet. The CFD model can be built
quickly if state-of-the-art software is used and the time needed to generate a
solution is modest. Consequently, it can be used to make cost savings by reducing
the amount of experimental testing needed to achieve a satisfactory design.
References
- B. F. Magnussen and B. W. Hjertager, "On Mathematical Modelling of
Turbulent Combustion with Special Emphasis on Soot Formation and Combustion,"
Sixteenth Symposium (International) on Combustion, pp. 719-729, The
Combustion Institute, Pittsburg, 1976.
- C. K. Westbrook and F. L. Dryer, "Simplified Reaction Mechanisms for
the Oxidation of Hydrocarbon Fuels in Flames," Combustion Science and
Technology, Vol. 27, pp. 31-43, 1981.