Case Study – Fume Dispersion from a Plasma Laboratory
The challenge
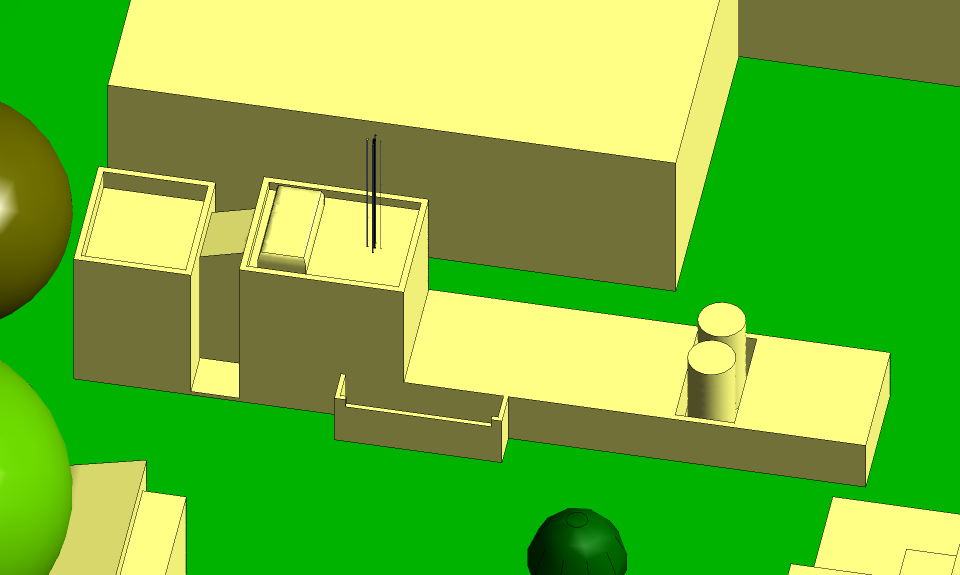
The University of Birmingham is refurbishing the Plasma Laboratory in the northwest
corner of the Edgbaston Campus. The southern tower of the Laboratory will be
fitted with four roof-top stacks arranged around a supporting mast, as shown in
Figure 1. For the present, only two of the stacks will be active. One will
discharge 100% hydrogen at 200°C to the atmosphere and the other will discharge
100% nitrogen at 20°C. The gases must disperse safely into the atmosphere and not
pose a risk to the occupants of other buildings or pedestrians.
Fig 1 Plasma Laboratory seen from the west
The solution
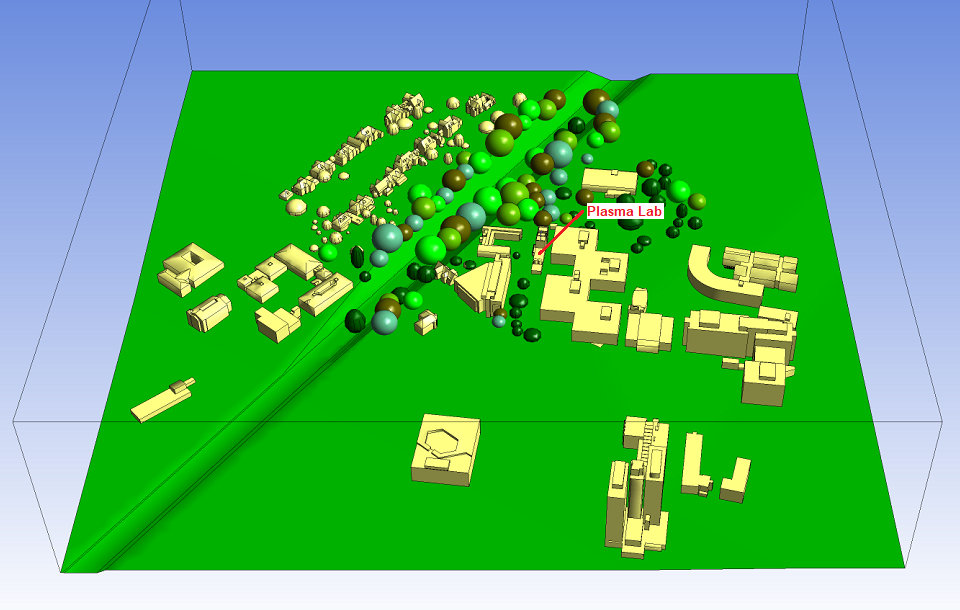
Atkinson Science carried out a computational fluid dynamics (CFD) study to
determine whether the gases would disperse safely, given the wind conditions
expected on the campus. The CFD model is shown in Figure 2. The model is centred
on the Plasma Laboratory and includes the buildings and landscape over an area of
600 m × 600 m.
Immediately to the northwest of the Laboratory, the landscape is densely populated
with trees. It is not possible to model the canopies of the trees in all there
detail and so they were represented as simple ellipsoids having the same volume as
the canopies. In summer the trees will be full of leaves and will shelter the
Plasma Laboratory from winds from the northwest quadrant. In winter the trees will
lose their leaves and offer very little shelter. To take account of this seasonal
change, we made two sets of computations for winds from the northwest quadrant: a
set of summer computations with the ellipsoids in place, and a set of winter
computations with the ellipsoids deleted from the model.
Fig 2 CFD model seen from the south
Hydrogen and air will form a combustible mixture if the concentration of hydrogen
in the air is between the lower and upper flammability limits for hydrogen-air
mixtures. At atmospheric pressure, the flammability limits based on volume percent
in air are 4.0 and 75.0. If the concentration of hydrogen is between these limits
then the mixture will burn if an ignition source (spark or flame) is present,
otherwise the mixture will remain stable unless the mixture temperature is allowed
to reach the auto-ignition temperature, in which case the mixture will
auto-ignite. For hydrogen-air mixtures the auto-ignition temperature is 500°C,
which is well above the temperature at which the hydrogen is generated and
discharged from the stack. The dilution factor is the ratio of the mass fraction
of hydrogen leaving the stack to the mass fraction downstream. We can show that at
the lower flammability limit the dilution factor is 346 and at the upper
flammability limit the dilution factor 5.79. The most important of these two
numbers is the first. Before the discharge from the stack reaches a building or
the ground, the dilution factor must exceed 346.
Any atmosphere with an oxygen level below 19.5% by volume is considered to be
oxygen deficient and dangerous to health. We can show that the dilution factor for
the discharge from the nitrogen stack must reach 14.9 before the discharge is
safe.
We used Met Office data to determine the wind conditions that are likely to occur
over the Edgbaston Campus. For the summer period we made computations at three
wind speeds (0.25, 2.5 and 10 m s−1) with the wind from the north and
every 45° from the north (24 computations). For the winter period we removed the
tree canopies to the northwest of the laboratory then repeated the computations
with the wind from the north, the northwest and the west and the same wind speeds
as for summer (9 computations). For each wind condition we plotted the isosurface
representing a dilution factor of 1000 for the hydrogen and for the nitrogen.
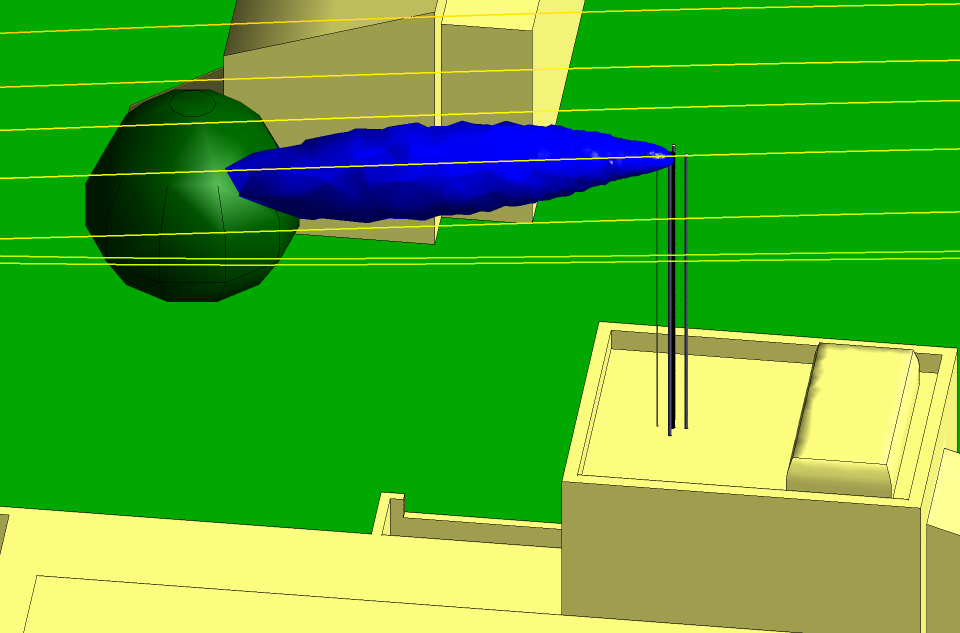
Figures 3 and 4 show the isosurfaces for the hydrogen and the nitrogen,
respectively, for the summer period with the wind from the north at
10 m s−1. The strong wind and the down-wash of air from the trees to
the north force the hydrogen and the nitrogen along a downward trajectory about
10° from the horizontal. The down-wash can be seen in the streamlines included in
the Figures. However, there is no possibility of the isosurfaces coming into
contact with a building or the ground and a dilution factor of 1000 is
considerably higher than is necessary to make the gases safe.
The hydrogen disperses much more quickly than the nitrogen and this is because the
hydrogen is a much lighter gas than the nitrogen and has much less upward
momentum. We can show that the upward momentum of the nitrogen is 22 times that of
the hydrogen.
Fig 3 Isosurface for the hydrogen in summer
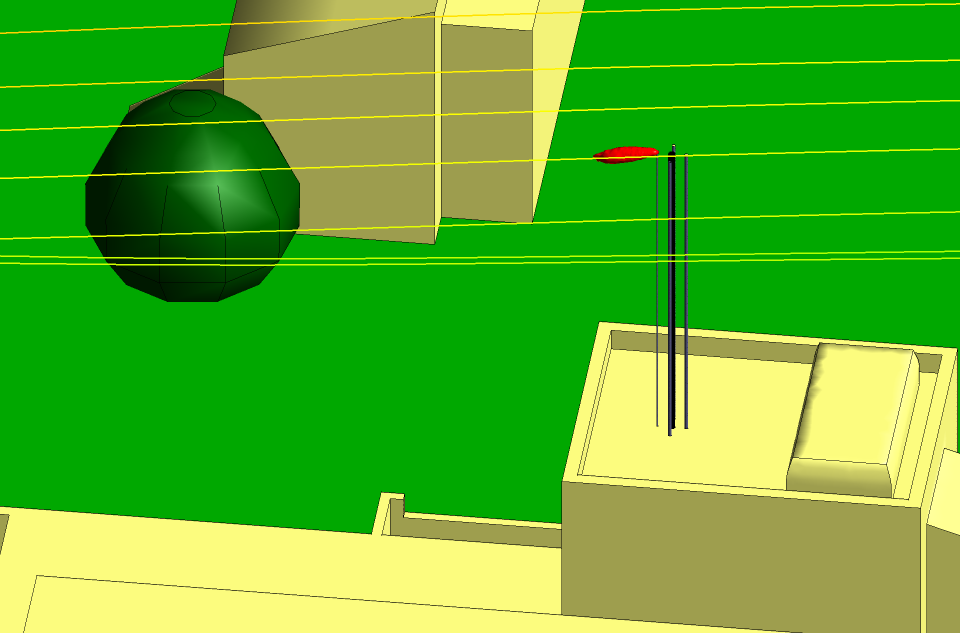
Fig 4 Isosurface for the nitrogen in summer
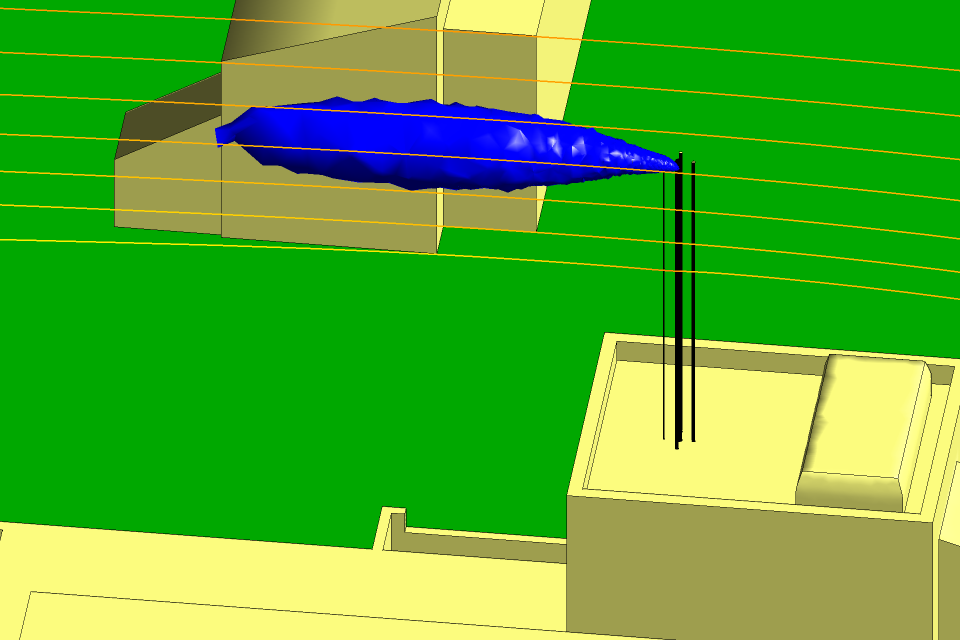
Figures 5 and 6 show the isosurfaces for the hydrogen and the nitrogen,
respectively, for the winter period with the wind from the north at
10 m s−1. The tree canopies have been removed from the CFD model so
there is no longer a down-flow of air and the hydrogen and the nitrogen follow a
horizontal trajectory. The Figures includes the flow streamlines, which show that
the flow is horizontal as it passes over the stacks. As in the summer period, the
gases are diluted to safe levels long before they can come into contact with a
building or the ground.
In fact in all 33 computations, we found that none of the isosurfaces came into
contact with a building or the ground. Thus for all of the wind conditions
considered over both the summer and winter periods we were able to say that the
system of stacks was perfectly safe.
Fig 5 Isosurface for the hydrogen in winter
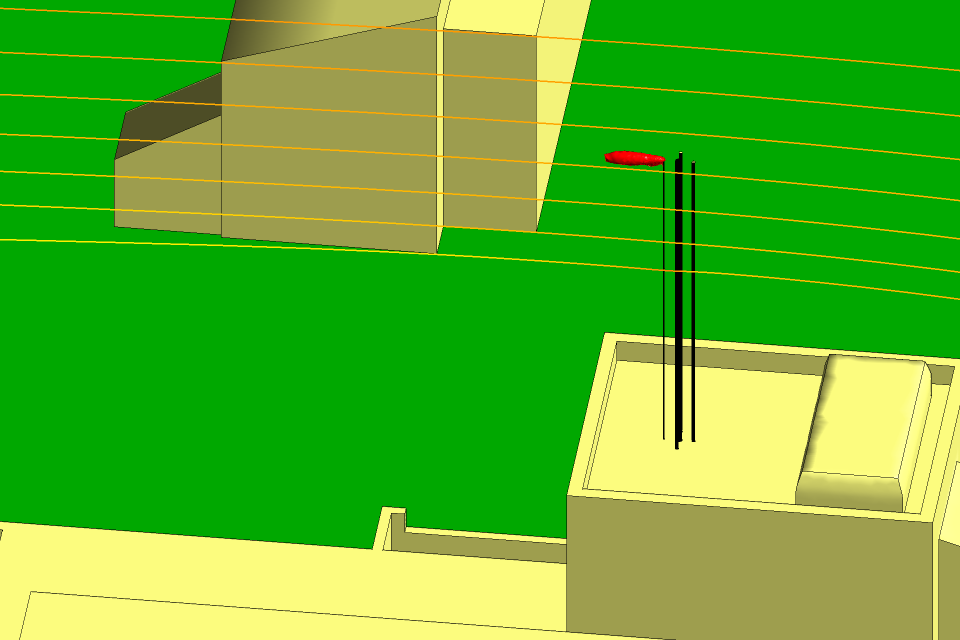
Fig 6 Isosurface for the nitrogen in winter
The benefits
The CFD study demonstrated to the University of Birmingham that the system of
stacks proposed for the Plasma Laboratory would perform satisfactorily in the wind
conditions likely to occur on the Edgbaston Campus.